In March 2014, the World Health Organization (WHO) reported an outbreak of Ebola virus disease in West Africa. With no previous outbreaks in this region, it had been three months from when the outbreak started until it was detected and by that time it had already spread to two other countries. From the start, the scale of this event tested the world’s ability to respond to and control an outbreak which would require coordination and collaboration of many international stakeholders.
Dr. Gary Kobinger and the special pathogens team at the Public Health Agency of Canada’s National Microbiology Laboratory (NML) in Winnipeg were well practiced to respond. Spearheaded by biologist Allen Grolla, they pioneered and refined a mobile laboratory system that they have used in more than a dozen outbreaks around the world since 2003. It was put to good use during the Ebola epidemic, with the NML sending 24 teams of experts to as many as three sites at a time and testing more than 5500 samples in total over 15 months.
Other preparations that had also been going on well before the outbreak started include the development of the Ebola experimental vaccine, known as VSV-EBOV and a treatment called ZMapp™ made up three different antibodies, two of which were discovered at the NML. Results from the testing of both products during this outbreak are extremely promising.
The process to create the vaccine involves inserting a single gene taken from the surface of the Ebola virus in place of a gene in an animal virus called vesicular stomatitis virus (VSV). When administered, the vaccine trains the immune system to react quickly and beat the infection. Even though it uses a component of the Ebola virus, the vaccine does not contain any live Ebola virus.
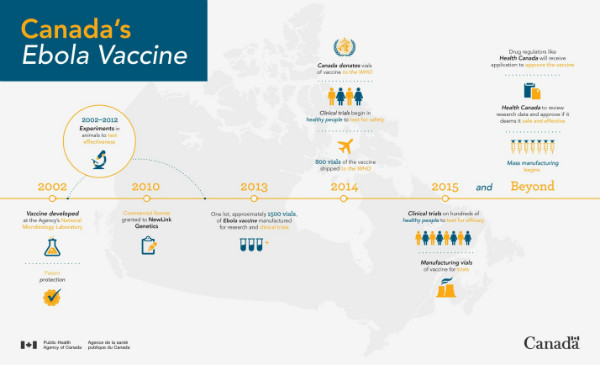
A timeline of the development of Canada’s Ebola Vaccine
Prior to the West Africa outbreak, studies by both the NML and American collaborators had progressed to a point where human grade vaccine was manufactured for human trials. By having this vaccine already on hand and donating it to the WHO and others for clinical trials, the Public Health Agency enabled trials to begin rapidly and eventually having a promising impact on the outbreak.
There are a number of trials continuing in Africa, one of which uses a ring vaccination model - when a patient is suspected of having Ebola, the medical team creates a “protective ring” of immunity by vaccinating everyone around the patient (friends, neighbours and family) to stop the transmission. The NML team continues to track the results of what is a promising vaccine.
What's next?
In 2010, the Public Health Agency of Canada licensed the vaccine to BioProtection Systems Corporation, a wholly owned subsidiary of NewLink Genetics Corporation. NewLink is now working with Merck and government regulatory agencies to get the vaccine cleared for routine use in situations of need.
The Ebola vaccine development is a concrete example of how Agency scientists are helping keep Canadians safe and healthy in a more complex and open world.
To learn more about the Ebola vaccine, visit the Agency’s website.
