Background
In 2018, the Governance Committee for Implementation of Government-Wide Scientific Integrity PolicyFootnote 1 (the Governance Committee), whose membership includes the Chief Science Advisor, the Secretary of the Treasury Board (TBS) and the President of the Professional Institute of the Public Service of Canada (PIPSC), published the Model Policy on Scientific Integrity.Footnote 2 The model was drafted as a guide for 24 federal departments and agencies that were required to implement a scientific integrity policy by December 31, 2019Footnote 3 pursuant to a 2016 Memorandum of Agreement between TBS and PIPSC. Between early December 2019 and late January 2020, the 24 implicated departments were surveyed to determine their progress in implementing departmental scientific integrity policies and procedures. As part of the survey, departments were asked to provide their own policy along with a detailed explanation of how it differed from the model policy. Subsequent progress was evaluated based on a follow-up survey conducted between early December 2020 and late January 2021.
The surveys
The 2019-20 and 2020-21 surveys asked departments to provide information on 13 compliance measures (Appendix A), each of which was associated with a non-discretionary provision of the model scientific integrity policy.Footnote 4 The 2020-2021 survey also included three additional questions by which departments and agencies could provide updates on implementation measures taken since the earlier survey. Since departments had discretion to adopt, adapt or even replace the model as they saw fit, provisions in the model policy that prescribe non-discretionary actions or activities (and associated compliance measures) would not be applicable if the department chose to eliminate the provision in question or render it discretionary. As the Office of the Chief Science Advisor (OCSA) is committed to evidence-based assessment of progress on scientific integrity, departments were asked to provide the evidence necessary to support each survey question response.
Results
The 2020-2021 survey was completed by 23 of 25 implicated departments or agencies. Upon inquiry, the remaining two provided some information on the status of their policies. As of January 30, 2021, 80% (20 of 25) of implicated departments and agencies had a scientific integrity policy in effect (Fig. 1).
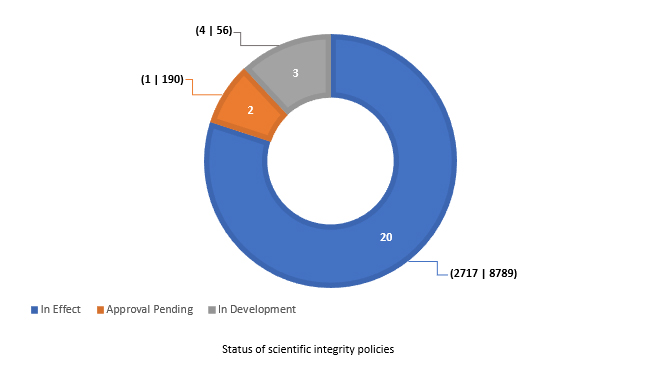
Fig. 1. The number of federal departments or agencies with scientific integrity policies in effect, pending approval, or in development as of January 30, 2021. Shown in parentheses are the number of employees in the RE (researchers) and SP (Applied Science and Patent Examination) occupational groups in the associated departments or agencies.Footnote 5
In support of responsible conduct of research, two key non-discretionary provisions of the model scientific integrity policy are the requirement for peer review of all technical communications and, where appropriate, approval of proposed research by a research ethics board.Footnote 6 Of the 20 departments and agencies with a scientific integrity policy in effect as of January 30, 2021, 15 (including 11,045 employees in the RE and SP groups) have peer review requirements in place, while the same number (15, accounting for 10,573 employees in the RE and SP groups) have, where appropriate, a requirement for research ethics board approval. Requiring peer review and, where appropriate, research ethics board approval was considered not applicable for one and two departments, respectively.
The model policy includes non-discretionary provisions facilitating the federal science and research community in providing advice on regulations and policy, on departmental research programs, and on prioritizing investments in research. To this end, as of January 2021, 15 of 20 (75%, accounting for 10,107 employees in the RE and SP groups) departments and agencies with a scientific integrity policy in effect have formal processes for soliciting advice on policies and regulations; 13 of 20 (65%), accounting for 9,661 employees in the RE and SP groups, have formal processes for soliciting advice on research programs; and 14 out of 20 (70%), accounting for 10,923 employees in the RE and SP groups, have formal processes for soliciting advice on the prioritization of departmental research investment. For three (15%) of responding departments, a formal process for soliciting researcher/scientist advice on prioritizing investment in research was considered not applicable.
As of January 30, 2021, all departments with scientific integrity policies in effect have notified their employees about the policy. Moreover, all departments whose departmental scientific integrity policies requires a Scientific Integrity Lead (SIL) have appointed one.
Since the 2019-2020 survey, departments and agencies have made significant progress implementing processes for reporting and recording instances of policy conflict or incompatibility with their scientific integrity policy; bringing forward and investigating breaches of scientific integrity; and soliciting advice on policies or regulations, research programs, and the prioritization of departmental research investment. Although significantly improved since the 2019-2020 survey, almost half of reporting departments have yet to implement processes for notifying external collaborators and contractors about scientific integrity policies (Fig. 2).
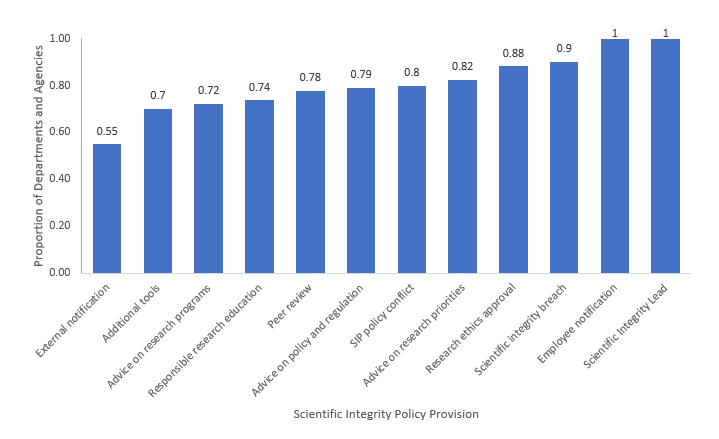
Fig. 2. The proportion of departments or agencies who have a scientific integrity policy in effect and for which the actions or activities corresponding to a particular non-discretionary provision in the model policy have been implemented as of January 30, 2021. Departments whose SIPs did not include the provision in question, or decided not to report the associated compliance measure in the 2020-2021 survey, have been excluded from the sample.Footnote 7 For a description of the listed non-discretionary scientific integrity provisions, see Appendix A.
Of the 20 departments and agencies with scientific integrity policies, none have amended their policy in the last year. Fourteen have produced additional procedures, policies, guidelines, tools, training or professional development opportunities relevant to their policies. In 6 of these 14 cases, these additions include the development and implementation of scientific integrity breach allegation and investigation processes. Other additions include the development of a dedicated scientific integrity policy resource page for departmental websites; additional training materials to support outreach to employees; and guidance for employees on scientific integrity issues such as peer review of technical communications, communication and dissemination of research and scientific information, and informing external contractors of the scientific integrity policy and encouraging them to comply with its provisions.
Has the model policy been successful?
The model policy was designed to facilitate a whole-of-government approach to scientific integrity. By developing a model based on extensive consultations, the Governance Committee was hopeful that departments and agencies would adopt the model with comparatively few modifications. And indeed, this has been the case: all 20 departmental policies currently in effect closely resemble the model, and many are virtually identical. Most substantive changes represent cases where departments have eliminated certain non-discretionary model provisions because, in their view, the provisions do not apply or are redundant given existing departmental processes and procedures. Perhaps the most significant change is the inclusion by a single department of a set of non-discretionary provisions designed to recognize and safeguard Indigenous knowledge and to ensure its due and appropriate consideration in decision-making.
Next steps
The 2019-2020 and 2020-2021 survey results clearly demonstrate that federal departments and agencies have made, and continue to make, considerable progress on scientific integrity. Over the next year, the Governance Committee will:
- Continue to monitor and evaluate scientific integrity policy compliance and assist departments in resolving outstanding issues, including especially those related to peer review of technical communications or research ethics board approval of departmental research;
- Explore with departments and agencies the implications of departmental scientific integrity policies on Open Science initiatives (and vice versa) to ensure consistency and complementarity;
- Work with departments and agencies to develop and provide education and training opportunities on a number of issues related to scientific integrity, especially the role of researchers and scientists in evidence-informed decision-making;
- Collaborate with departments and agencies to develop and deploy an employee survey to gather some of the information that will enable departments to undertake performance evaluation of their scientific integrity policies.
- Explore with departments and agencies the possibility of revising existing scientific integrity policies to include provisions for safeguarding, communicating and appropriately using Indigenous knowledge.
Though much work still remains, the Governance Committee is pleased with the progress made by federal departments and agencies on scientific integrity, especially in light of current urgent demands and associated resource reallocations arising from the COVID-19 pandemic. The committee is keenly aware that this progress reflects the commitment of federal departments and agencies, ministers and employees to ensuring that government decisions are informed by scientific evidence, and that Canadians are informed about important scientific issues that affect them.Footnote 8
Appendix A: Scientific Integrity Policy (SIP) Compliance Survey Questions
CM1. What is the status of your organization’s SIP?
CM2. Has your organization notified employees about the SIP?
CM3. Has your organization implemented additional procedures policies, guidelines, tools, training or professional development opportunities in support of the SIP?
CM4. Does your organization have a process for reporting and recording instances of policy conflict or incompatibility with the SIP?
CM5. Does your organization require that all technical communications undergo peer review?
CM6. Does your organization require that, where appropriate, research or scientific projects be reviewed and approved by a Research Ethics Board (REB)?
CM7. Has your organization taken steps to notify/inform contractors or extramural collaborators of the SIP and encourage them to comply with its articles?
CM8. Has your organization appointed a Science Integrity Lead?
CM9. Does your organization have a process for bringing forward and investigating breaches of scientific integrity?
CM10. Does your organization have a mechanism and/or procedure for soliciting researcher/scientist advice on deartmental policy and/or regulations?
CM11. Does your organization have a mechanism and/or procedure for soliciting researcher/scientist advice on its research programs?
CM12. Does your organization have a mechanism and/or procedure for systematically soliciting researcher/scientist assistance in identifying and prioritizing federal investment in research?
CM13. Does your organization have measures in place to support education, training and/or professional development in any of the following areas: responsible conduct in research; research eithcs; and the annotation, management and archiving of research and scientific data?
2020-2021 Survey Additional Questions:
- Did your organization complete the 2019 compliance survey?
- Have there been any amendments to your organization’s Scientific Integrity Policy (SIP) since the 2019 compliance survey?
- Has your organization developed any additional procedures, policies, guidelines, tools, training or professional development opportunities relevant to the SIP since the 2019 survey?
